Pharmaceutical ingredients undergo stringent testing guidelines to meet tough quality standards. In rare occasions, passing USP compendial testing cannot guarantee the pharmaceutical ingredients are not tainted with foreign contaminants.
In this blog, we will focus on the identification of particulate contamination in pharmaceutical products. This is an example where several batches of pharmaceutical raw materials passed the USP compendial tests but received numerous customer complaints due to particulate contamination.
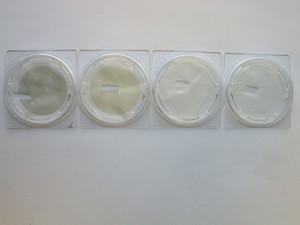
The particulate was first isolated from the raw material by dissolution of the product followed by filtration. In Figure 1, the two filters to the right are lab blanks and the two to the left are raw material samples. You can clearly see the filters on the left are tainted. Figure 2 shows a closer look of the filters by light microscopy; the filters are loaded with extraneous particles of various colors, shapes and sizes.
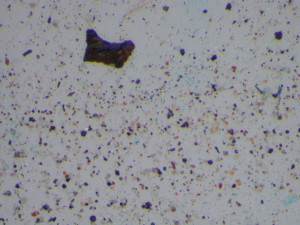
After the initial documentation and examination of the filters by light microscopy, we begin to isolate representative particulate for identification. We will illustrate a few examples here. Black particles (Figure 3) were prevalent on the filters, so we isolated a couple and analyzed them by Raman spectroscopy. Raman spectroscopy is great for analyzing small particles (down to one micrometer). The technique is also non-destructive if you don’t burn the sample by ramping up the laser power. Figure 4 shows a Raman spectrum of a black particle; the spectrum is consistent with a charred carbon.

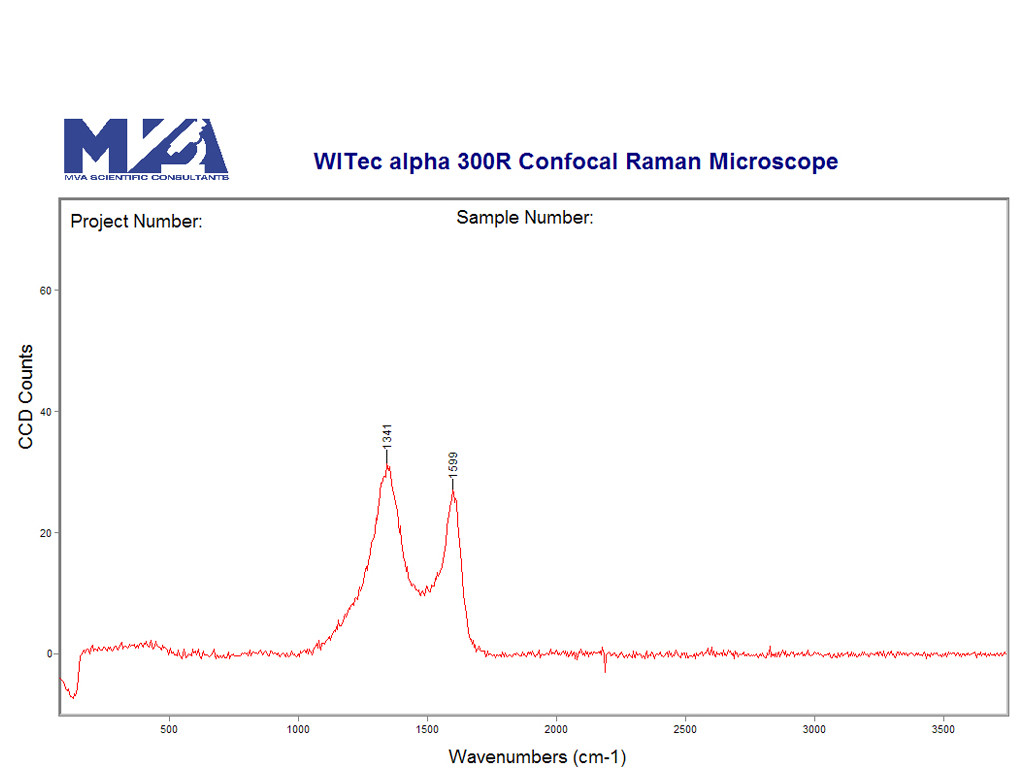
Figure 5 shows a filter with an extraneous fiber in addition to the small particles. Examination of the fiber by polarized microscopy determined it to be consistent with a human hair (Figure 6). Human hair is one of the most common particulate contaminants that occur in pharmaceutical raw material.
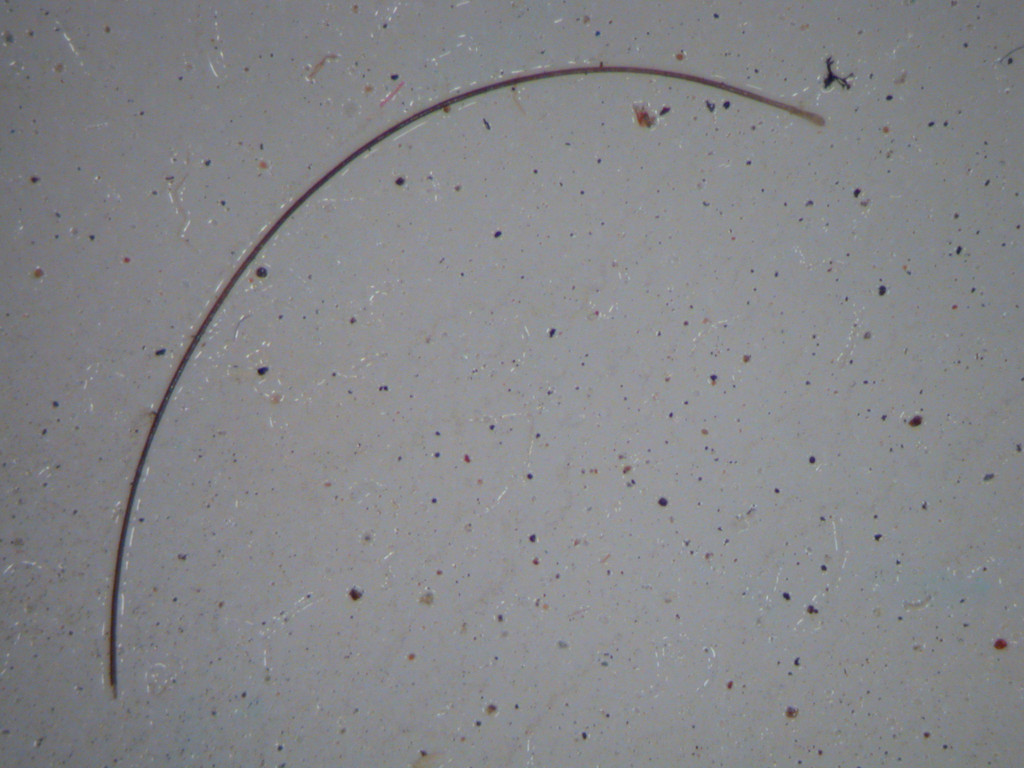
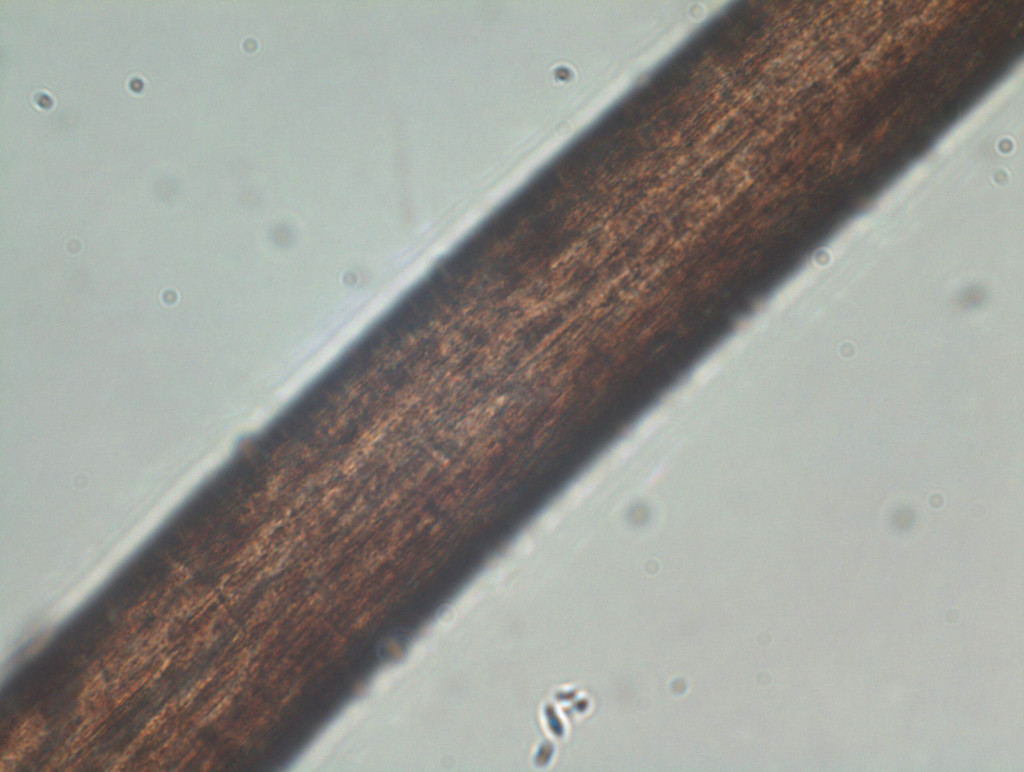
Figure 7 shows a scanning electron microscopy (SEM) image of one of the black particles isolated from the filter. SEM analysis is great for looking at the surface morphology and texture of small particles. Energy dispersive x-ray analysis shows that this particle is consistent with an iron oxide (Figure 8). We suspect it is a corrosion product.

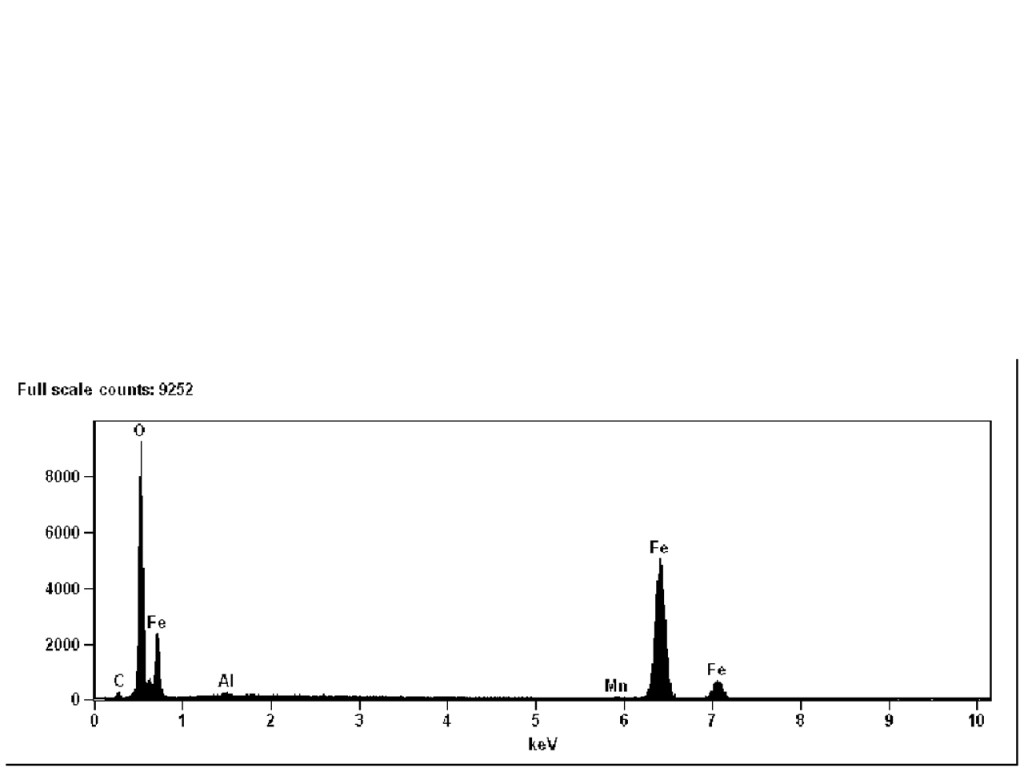
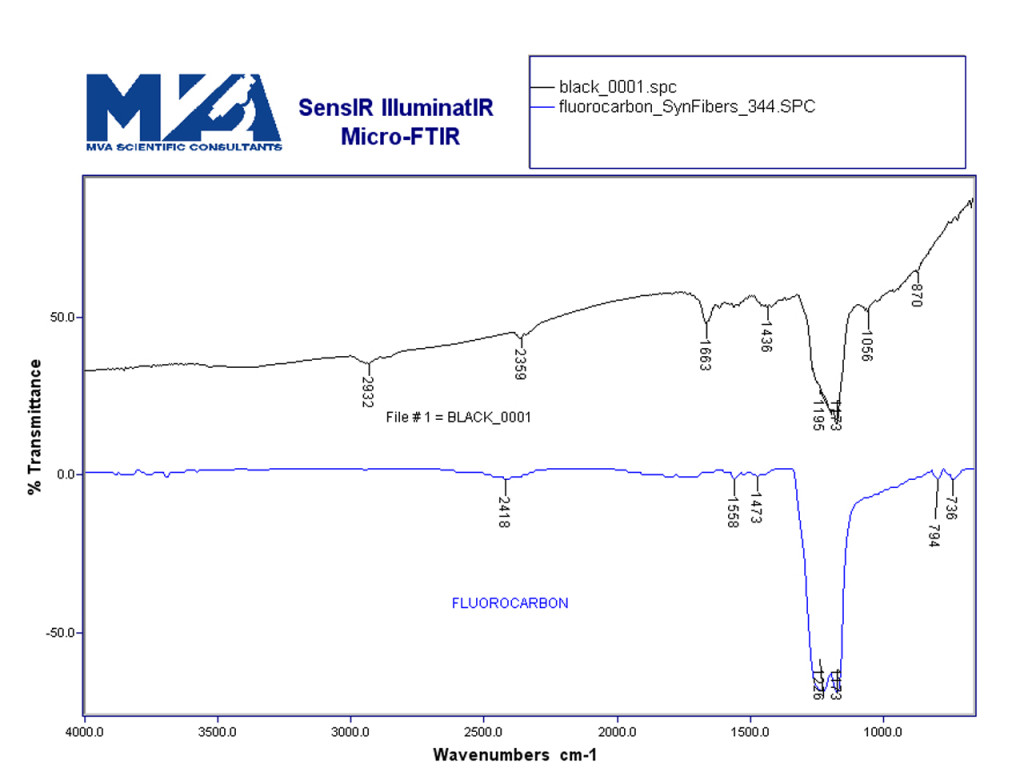
Figure 9 shows an SEM image of some polymeric material. Analysis by FTIR shows that it is consistent with a fluorocarbon polymer (Figure 10). FTIR is a great instrument for identifying organic materials. It is non-destructive and gives molecular information to help quickly identify unknown materials.

Analysis of the contaminants in the raw materials by SEM, light microscopy, FTIR and Raman showed a large variety of materials including several different polymers, metals and metal corrosions, inorganic salts, combustion products, charcoal, textile fibers and hair. The investigation revealed that the particles originated from multiple sources.
For more information please contact us at 770-662-8509 or info@mvainc.com.




