Expand Your Knowledge
Our resource center archives our case studies, published articles, blogs, webinars, and image galleries. Discover ways microscopy has made a meaningful impact.
MVA Scientific Consultants has developed a customized and systematic approach for glass delamination analysis which includes special sample preparation, filtration, and examination by light microscopy and SEM-EDS. Our methodology is beneficial in promptly providing you valuable insights to the nature of the occurrence.
In the pharmaceutical industry, glass delamination is defined as thin glass flakes shedding from the internal diameter of glass containers. The flakes, also called “glass lamellae,” can be shed and suspended in parenteral drug products. This is a major concern in the pharmaceutical industry and has resulted in a number of recalls. Detecting glass lamellae is one of the key aspects of glass delamination analysis. USP laid out some analytical guidelines for pharmaceutical manufacturers and analytical laboratories on how to screen for glass delamination.
Examination of internal diameter of glass container
USP <1660> recommends three key parameters for screening glass delamination in glass containers. The parameters are the condition of the glass surface as received, the presence of extracted elements in solution, as well as the presence of visible and subvisible glass particles.
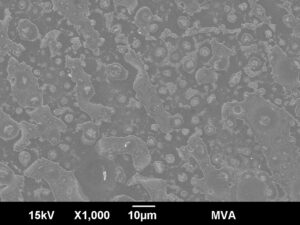
Example of a delaminated glass vial surface analyzed by SEM-EDS
Analytical approach for examination of glass container
MVA Scientific Consultants has customized a systematic approach for glass delamination analysis which includes special sample preparation, filtration, and examination by light microscopy and SEM-EDS.

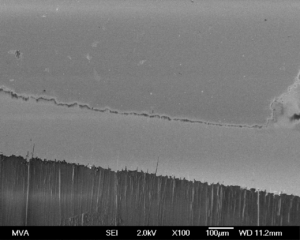
SEM image of glass corrosion
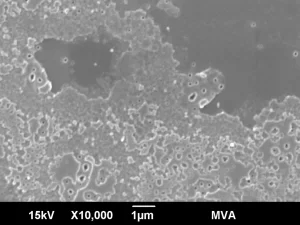
High magnification SEM imaging showing the pits and surface structure of the interior glass surface
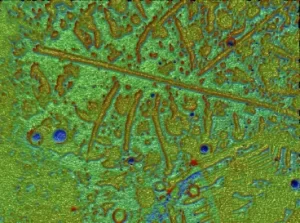
Surface roughness profile of the glass interior showing the pits and delaminated area of the surface

Glass lamellae observed by light microscopy
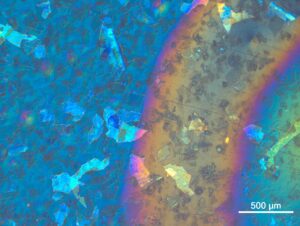
DIC image of glass lamella on filter
To learn more about how we can help you with glass delamination analysis, call us at 770-662-8509 or email info@mvainc.com.